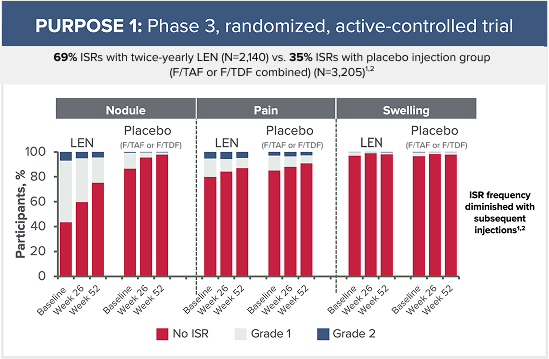
Grade 3 ISRs reported in PURPOSE 1 included:2
- 6 cases of injection-site ulcer (3 in the LEN for PrEP group, 2 in the F/TAF group, and 1 in the F/TDF group)
- 1 case of nodule (in the LEN for PrEP group)
- 1 case of pain (in the F/TDF group)

This medical site is intended as an online educational resource for U.S. healthcare professionals only. This site may include references to scientific information about Gilead products or uses that have not been approved by the U.S. Food and Drug Administration. The information provided is not intended to be and should not be understood to provide medical advice.
By clicking “I am a U.S. healthcare professional”, you are acknowledging that you are a healthcare professional licensed to practice in the U.S.
LEN for PrEP (marketed as YEZTUGO) is a human immunodeficiency virus type 1 (HIV-1) antiretroviral agent with long-acting properties indicated for pre-exposure prophylaxis (PrEP) to reduce the risk of sexually acquired HIV-1 in adults and adolescents weighing at least 35 kg (77 lb) who have a higher likelihood of HIV-1 acquisition. Individuals must have a negative HIV-1 test prior to initiating LEN for PrEP.
LEN for PrEP is administered in a two-step dosing regimen. It starts with a combination of subcutaneous injections and oral tablets, followed by continuous subcutaneous injections once every 6 months (26 weeks) ± 2 weeks.
BOXED WARNING: RISK OF DRUG RESISTANCE WITH USE OF LEN for PrEP FOR HIV-1 PRE-EXPOSURE PROPHYLAXIS (PrEP) IN UNDIAGNOSED HIV-1 INFECTION
Individuals must be tested for HIV-1 infection prior to initiating LEN for PrEP, and with each subsequent injection of LEN for PrEP, using a test approved or cleared by the FDA for the diagnosis of acute or primary HIV-1 infection. Drug-resistant HIV-1 variants have been identified with the use of LEN for PrEP by individuals with undiagnosed HIV-1 infection. Do not initiate LEN for PrEP unless negative infection status is confirmed. Individuals who acquire HIV-1 while receiving LEN for PrEP must transition to a complete HIV-1 treatment regimen.
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE DRUG REACTIONS
To report SUSPECTED ADVERSE DRUG REACTIONS, contact Gilead Sciences, Inc. at 1-800-GILEAD-5 or FDA at 1-800-FDA-1088 and select option #3 or www.fda.gov/medwatch.
DRUG INTERACTIONS
REFERENCE:
The efficacy and safety of LEN for PrEP in reducing the risk of HIV-1 acquisition were evaluated in two randomized, double-blind, active-controlled, multinational trials: PURPOSE 1 and PURPOSE 2.1
PURPOSE 1:
Study Design
Baseline Characteristics
Outcomes/Results
Overall HIV-1 Incidence Outcomes in PURPOSE 1 at Final Analysis1a
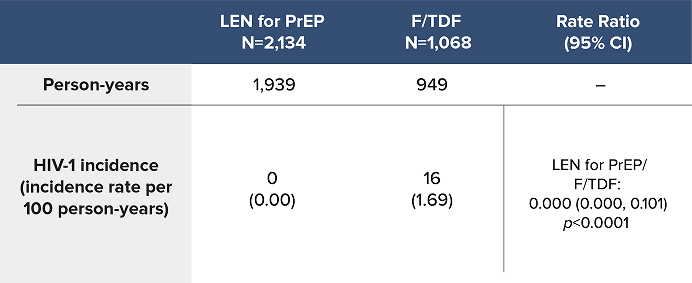
LEN for PrEP demonstrated superiority with a 100% reduction in the likelihood of incident HIV-1 acquisition over F/TDF1
PURPOSE 2:
Study Design
Baseline Characteristics
Outcomes/Results
Overall HIV-1 Incidence Outcomes in PURPOSE 2 at Final Analysis1a
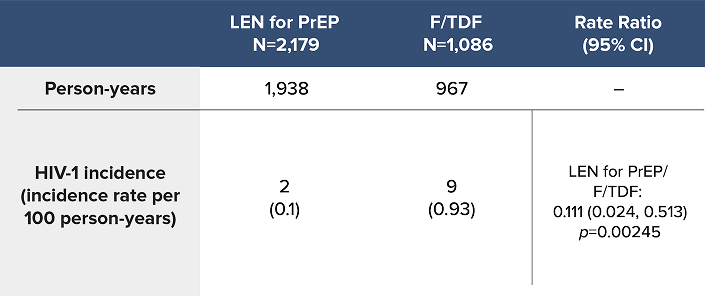
LEN for PrEP demonstrated superiority with an 89% reduction in the likelihood of incident HIV-1 acquisition over F/TDF1
LEN for PrEP Safety Data1
Grade 3 ISRs reported in PURPOSE 1 included:2
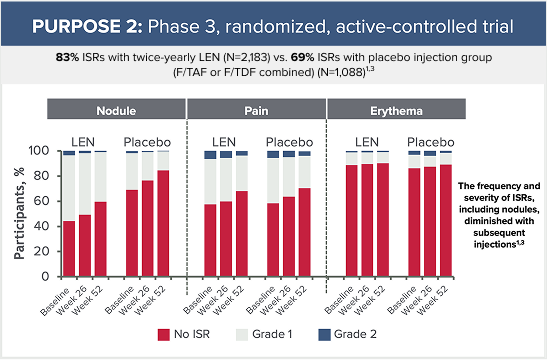
Grade 3 ISRs reported in PURPOSE 2 included:3
Most Common Non-ISR Adverse Drug Reactions (ADRs)
Reported in ≥2% of Participants Receiving LEN for PrEP in PURPOSE
1 or PURPOSE 2:1

HIV-1=human immunodeficiency virus type 1; LEN for PrEP=lenacapavir for pre-exposure prophylaxis.
REFERENCES:
Indications and Usage
LEN for PrEP is indicated for pre‑exposure prophylaxis (PrEP) to
reduce the risk of sexually acquired HIV-1 in adults and adolescents
weighing at least 35 kg who have a higher likelihood of HIV-1
acquisition. Individuals must have a negative HIV-1 test prior to
initiating LEN for PrEP.
HIV-1 Screening
Screen all individuals for HIV-1 prior to initiating LEN for PrEP, prior to each subsequent injection of LEN for PrEP, and additionally as clinically appropriate, using a test approved or cleared by the FDA for the diagnosis of acute or primary HIV-1 infection. When screening for HIV-1 prior to initiating LEN for PrEP, if an antigen/antibody-specific test is used and provides negative results, then such negative results should be confirmed using an RNA-specific assay, even if the results of the RNA-assay are available after LEN for PrEP initiation. When screening for HIV-1 prior to continuing LEN for PrEP, negative results from a rapid, point-of-care antigen/antibody test should be confirmed using a more sensitive assay.
General considerations when prescribing LEN for PrEP:*
Considerations for specific populations
Pregnancy
Lactation
Pediatrics
Geriatrics
Renal impairment
Hepatic impairment
REFERENCE:
LEN for PrEP is supplied as a combination of oral tablets and subcutaneous injections.

Each bottle contains 4 tablets:

Each injection dosing kit contains all the necessary components for injecting one complete dose (two 1.5 mL injections) of YEZTUGO:
HIV-1=human immunodeficiency virus type 1; LEN for PrEP=lenacapavir for pre-exposure prophylaxis.
REFERENCE:
Drugs without Clinically Significant Interactions with LEN
for PrEP
Based on drug interaction studies conducted with LEN for PrEP, no clinically significant drug interactions have been observed with:
Effect of Other Drugs on LEN for PrEP
Lenacapavir is a substrate of P-gp, UGT1A1, and CYP3A.
Effect of LEN for PrEP on Other Drugs
CYP3A=cytochrome P450 3A; HIV-1=human immunodeficiency virus type 1; LEN for PrEP=lenacapavir for pre-exposure prophylaxis; P-gp=permeability glycoprotein; UGT1A1=uridine 5’-diphospho-glucuronosyltransferase 1A1.
REFERENCE:
Algorithm intended for illustrative purposes only. This
information does not constitute medical advice.
The decision to start PrEP should be based on healthcare
professional judgment after shared clinical decision-making and
based on individual interest.
REFERENCES:
The LEN for PrEP dosing schedule consists of a required initiation dosing (subcutaneous injections and oral tablets) followed by once-every-6-months (26 weeks) ± 2 weeks continuation dosing (subcutaneous injections).1
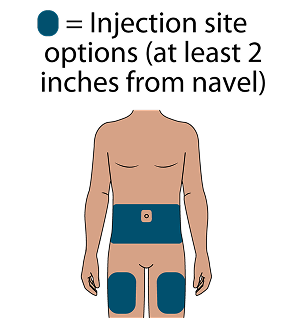
Subcutaneous (SC) injections are administered into the abdomen by a healthcare provider. The thigh may be used as an alternate site if preferred. Do NOT administer intradermally due to the risk of serious injection site reactions.1
An oral initiation dose is needed on Day 1 and Day 2 to achieve adequate concentrations of lenacapavir quickly due to the slow initial release of subcutaneous lenacapavir.1,2
Dosing Schedule for LEN for PrEP Initiation and Continuation in Adults and Adolescents Weighing at Least 35 kg1
927 mg by subcutaneous injection
(2 x 1.5 mL injections) and
600 mg orally (2 x 300 mg tablets)

600 mg orally
(2 x 300 mg tablets)

927 mg by subcutaneous injection
(2 x 1.5 mL injections)

Missed Doses1
Missed Oral Initiation Dose
Anticipated Delayed Injections1
Dosing Schedule for Anticipated Delayed Injections: Weekly Oral Dosage1
Dosage of LEN for PrEP
Oral dosage of 300 mg taken once every 7 days.a
Resume the continuation injection dosage within 7 days after the
last oral dose.
Missed Injections1
Dosing Schedule After Missed Injections1
Dosage of LEN for PrEP
Reinitiate with initiation dosing schedule from Day 1 and then
continue with continuation injection dosing.

927 mg by subcutaneous injection
(2 x 1.5 mL injections)
every 26 weeks (+/- 2 weeks)
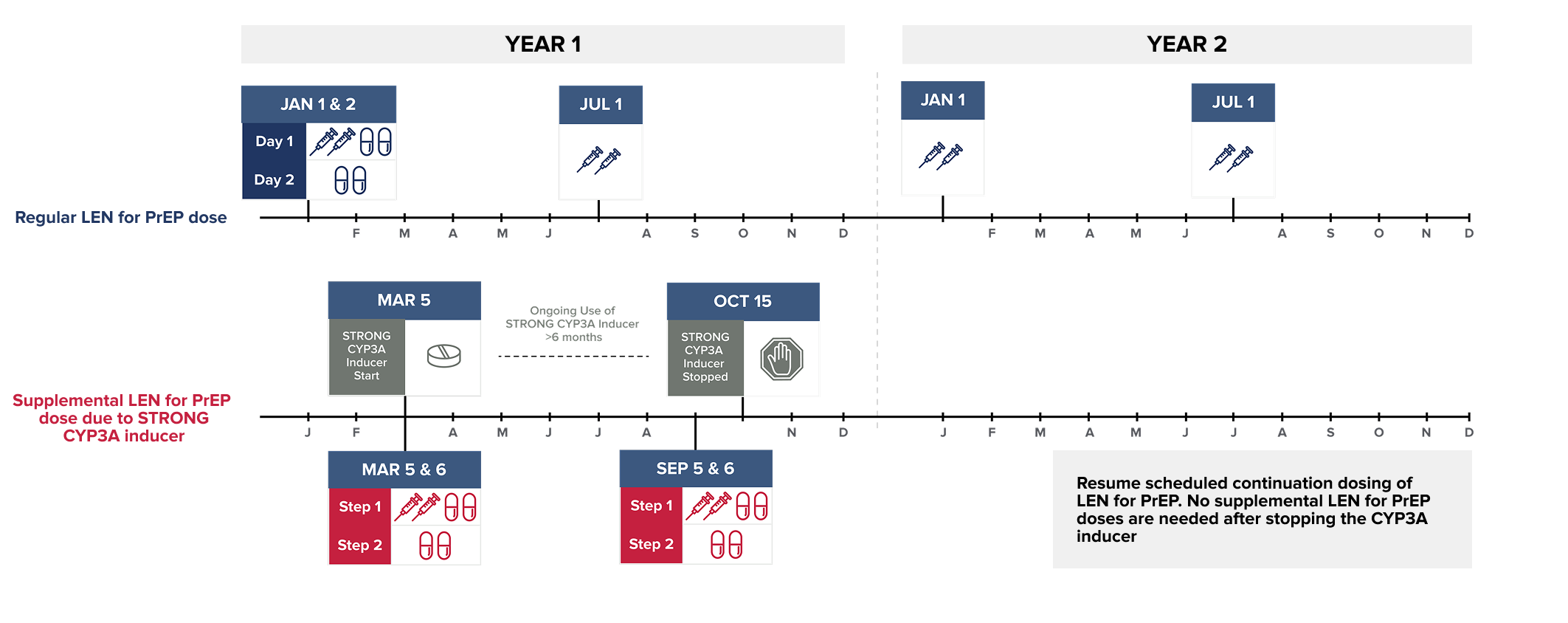
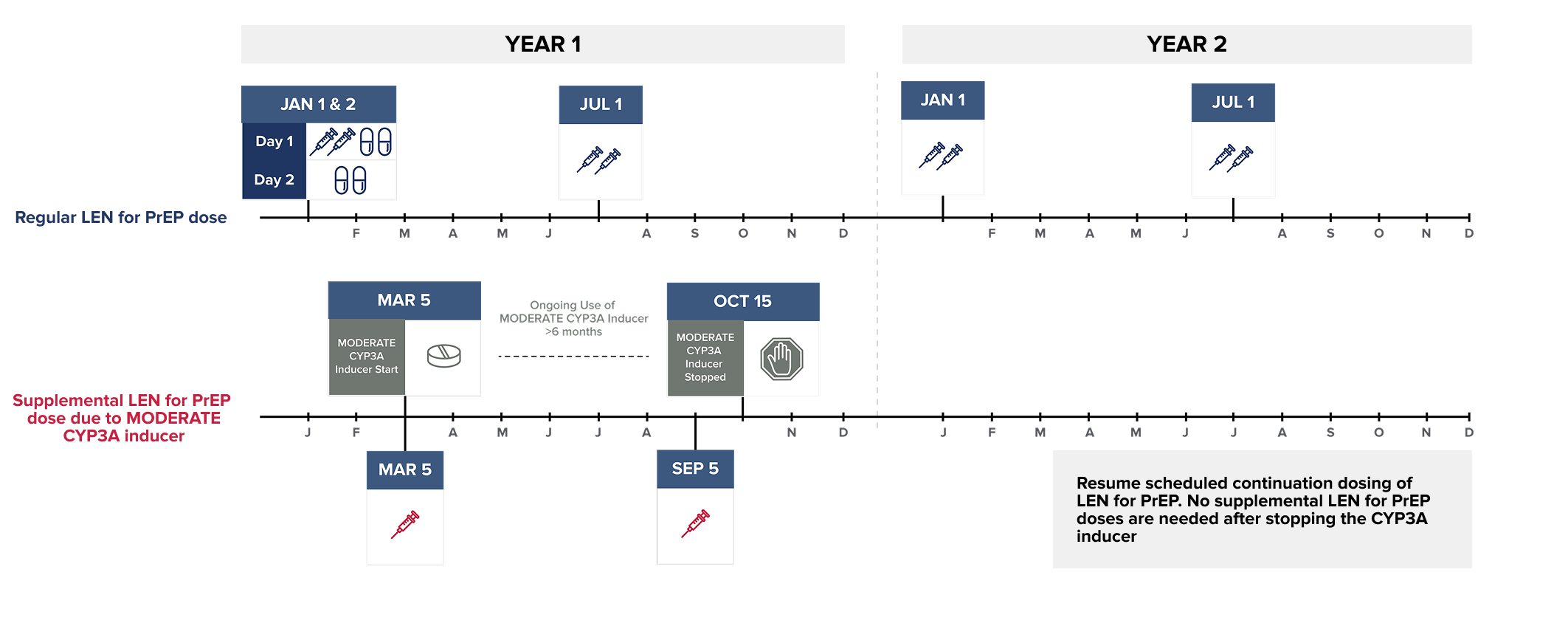
Wait at least 2 days after LEN for PrEP is first initiated before initiating STRONG CYP3A inducers. MODERATE CYP3A inducers may be started at any time after LEN for PrEP initiation.
NOTE: The STRONG CYP3A inducer may be initiated any time after 2 days following LEN for PrEP initiation.
Dosing recommendations are not available for the initiation of LEN for PrEP in individuals already receiving STRONG CYP3A inducers.
NOTE: The MODERATE CYP3A inducer may be initiated any time after LEN for PrEP is initiated.
Dosing recommendations are not available for the initiation of LEN for PrEP in individuals already receiving MODERATE CYP3A inducers.
REFERENCES:
LEN for PrEP Dosing Schedule Tool
Regular LEN for PrEP Dosing
Please select the type of LEN for PrEP dose:
Supplemental LEN for PrEP Dosing
Supplemental doses of LEN for PrEP are recommended in individuals starting a strong or moderate CYP3A inducer. Supplemental doses are given in addition to scheduled continuation LEN for PrEP doses, for as long as the individual remains on the CYP3A inducer.1
To see the recommended supplemental LEN for PrEP dose, select the CYP3A inducer from the list below:*1-4
Please select the date the regular injection dose of LEN for PrEP was administered.
| Day 1 initiation dose of LEN for PrEP on |
2 x 1.5 mL injections 2 x 300 mg tablets |
| Day 2 initiation dose of LEN for PrEP on | 2 x 300 mg tablets |
| Next continuation dose of LEN for PrEP on (+/- 2 weeks) | 2 x 1.5 mL injections |
| Continuation dose of LEN for PrEP on | 2 x 1.5 mL injections |
| Next continuation dose of LEN for PrEP on (+/- 2 weeks) | 2 x 1.5 mL injections |
Start of began on :
| Supplemental LEN for PrEP dose due to on | 1 x 1.5 mL injection |
Since continues for more than 6 months:
| Supplemental LEN for PrEP dose due to on (+/- 2 weeks) | 1 x 1.5 mL injection |
Continue to administer supplemental LEN for PrEP doses
every 26 weeks (+/- 2 weeks) from the original supplemental
dose.1
| Day 1 supplemental LEN for PrEP dose due to on |
2 x 1.5 mL injections 2 x 300 mg tablets |
| Day 2 supplemental LEN for PrEP dose due to on | 2 x 300 mg tablets |
Since continues for more than 6 months:
| Day 1 supplemental LEN for PrEP dose due to on (+/- 2 weeks) |
2 x 1.5 mL injections 2 x 300 mg tablets |
| Day 2 supplemental LEN for PrEP dose due to immediately following Day 1 | 2 x 300 mg tablets |
Continue to administer supplemental LEN for PrEP doses
every 26 weeks (+/- 2 weeks) from the original supplemental
dose.1
Dosing recommendations are not available for the initiation of LEN for PrEP in individuals already receiving strong or moderate CYP3A inducers nor in individuals receiving the weekly oral dose of LEN for PrEP.1
Dosing recommendations are available when strong CYP3A inducers are initiated starting at least 2 days after LEN for PrEP is first initiated; moderate CYP3A inducers may be started any time after LEN for PrEP is first initiated.1
Dosing recommendations are not available for the initiation of LEN for PrEP in individuals already receiving strong or moderate CYP3A inducers nor in individuals receiving the weekly oral dose of LEN for PrEP.1
Dosing recommendations are available when strong CYP3A inducers are initiated starting at least 2 days after LEN for PrEP is first initiated; moderate CYP3A inducers may be started any time after LEN for PrEP is first initiated.1
REFERENCES:
Individuals receiving LEN for PrEP should be informed about what to expect during and after injections.1
1. Drug depot formation

2. ISRs
3. None of the ISRs in the PURPOSE 1 and PURPOSE 2 trials were serious. Individual results may vary. Advise individuals receiving LEN for PrEP to tell their healthcare provider if they experience any ISRs or other side effects.1
Techniques to Help Address Pain
These techniques for injection site pain are commonly used in routine clinical care and are not specific to LEN for PrEP. Some individuals may find these techniques helpful.
If clinically appropriate, applying topical analgesics (e.g., lidocaine-prilocaine EMLA cream, not patches) can help reduce injection-related pain.7,8
Apply at least 30 minutes before injection to both injection sites, if not contraindicated.7,8
Before injecting, wipe off the cream from the injection site with an alcohol swab.5
REFERENCES:
Administer LEN for PrEP tablets orally, with or without food.1
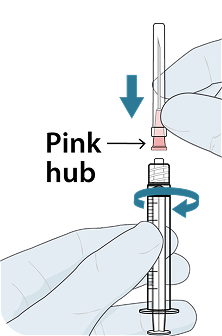
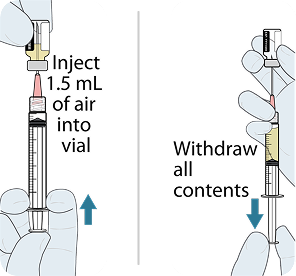
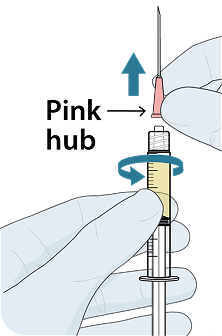
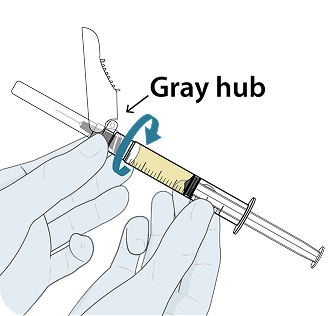
Administer LEN for PrEP injections subcutaneously. LEN for PrEP injection is only for SC administration into the abdomen by a healthcare provider. The thigh may be used as an alternate site if preferred.1
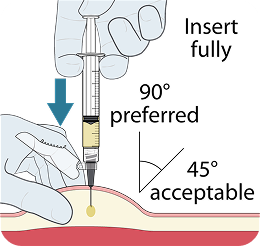
Injecting too superficially could lead to improper administration into the dermis. Intradermal injection of lenacapavir has been associated with serious ISRs, including necrosis and ulcer. Ensure LEN for PrEP is only administered subcutaneously.1
YEZTUGO injection is supplied in a single-use dosing kit (NDC 61958-3402-1), containing all the necessary components for two 1.5 mL injections (one complete dose), including withdrawal and injection needles.
YEZTUGO Injection Kit Components



NOTE: All components are for single use.
If the needle becomes damaged or unusable in some way (e.g., dropped on the floor or otherwise contaminated), that needle should not be used. Contact Gilead Sciences to request a replacement needle.2

REFERENCES:
The injection site should not be massaged or subjected to pressure post-injection.3
A small amount of drug leakage may occur.2-4 Leakage may be absorbed by small gauze.5
LEN has a bright yellow color so a relatively small amount of drug leakage could appear more noticeable.1
These post-injection care strategies are not specific to LEN for PrEP.
REFERENCES:

REFERENCE:
To help ensure correct administration technique, here are some key points to consider:
REFERENCE:
The most common adverse drug reactions (all grades) reported in at least 5% of participants receiving LEN for PrEP in either PURPOSE 1 or PURPOSE 2 were ISRs, headache, and nausea.1
Adverse Drug Reactions (ADRs) Reported in ≥2%a of Participants Receiving LEN for PrEP in PURPOSE 1 and PURPOSE 21a

Injection-site Reactions (All Grades) Reported in ≥2% of Participants Receiving LEN for PrEP in PURPOSE 1 and PURPOSE 21a

Grade 3 ISRs were reported in:1
None of the ISRs in PURPOSE 1 and PURPOSE 2 trials were serious.
To report SUSPECTED ADVERSE DRUG REACTIONS, contact Gilead Sciences, Inc. at 1-800-GILEAD-5 or FDA at 1-800-FDA-1088 and select option #3, or www.fda.gov/medwatch.
REFERENCES:
LEN for PrEP as Part of an Overall HIV-1 Prevention Strategy1
Supporting Individuals with HIV-1 Prevention1
REFERENCE:
Pregnancy1
Clinical Considerations
Risk Summary
Pregnancy Exposure Registry
Lactation1
REFERENCE:
Drug-Drug Interactions1
Discontinuing LEN for PrEP1
REFERENCE:
Potential Risk of Developing Resistance to LEN for PrEP1
Routine Testing to Minimize the Risk of Resistance1
REFERENCE:
REFERENCE:
LEN for PrEP adherence and persistence are crucial for achieving optimal outcomes.1
Importance of Staying on Schedule1
Advise individuals to stay under the care of a healthcare provider while receiving LEN for PrEP.
Emphasize that it is important to:
Emphasize the importance of receiving LEN for PrEP as scheduled because:
If an individual is unable to attend a scheduled 6-month (26 weeks) ± 2 weeks injection appointment:
Some things to consider before booking an individual’s 6-month (26 weeks) ± 2 weeks LEN for PrEP injection appointment:
Anticipated Delayed Continuation Injections1
Missed Injections1
Missed Oral Initiation Dose1
HIV-1=human immunodeficiency virus type 1; LEN for PrEP=lenacapavir for pre-exposure prophylaxis; STIs=sexually transmitted infections.
REFERENCES:
HIV-1=human immunodeficiency virus type 1; LEN for PrEP=lenacapavir for pre-exposure prophylaxis.
REFERENCE:
For access and support resources, including information on Advancing Access and acquisition of LEN for PrEP, visit PrEP.AdvancingAccess.com.
These highlights do not include all the information needed to use LEN for PrEP safely and effectively. See full prescribing information for YEZTUGO.
YEZTUGO® (lenacapavir) tablets, for oral use
YEZTUGO® (lenacapavir) injection, for subcutaneous use
BOXED WARNING: RISK OF DRUG RESISTANCE WITH USE OF LEN for PrEP FOR HIV-1 PRE-EXPOSURE PROPHYLAXIS (PrEP) IN UNDIAGNOSED HIV-1 INFECTION
Individuals must be tested for HIV-1 infection prior to initiating LEN for PrEP, and with each subsequent injection of LEN for PrEP, using a test approved or cleared by the FDA for the diagnosis of acute or primary HIV-1 infection. Drug-resistant HIV-1 variants have been identified with the use of LEN for PrEP by individuals with undiagnosed HIV-1 infection. Do not initiate LEN for PrEP unless negative infection status is confirmed. Individuals who acquire HIV-1 while receiving LEN for PrEP must transition to a complete HIV-1 treatment regimen.
927 mg by subcutaneous injection (2 x 1.5 mL injections) every 6 months (26 weeks) from the date of the last injection +/-2 weeks.
To report SUSPECTED ADVERSE DRUG REACTIONS, contact Gilead Sciences, Inc. at 1-800-GILEAD-5 or FDA at 1-800-FDA-1088 and select option #3, or www.fda.gov/medwatch.
Please see YEZTUGO full Prescribing Information, including Boxed Warning.
LEN for PrEP=lenacapavir for HIV-1 pre-exposure prophylaxis.
REFERENCE:

By clicking this link, you will be leaving the Gilead Medical Affairs Website and will be redirected to a third-party site. We do not control, endorse, or influence the claims or comments in the following third-party website.
To discuss a question with a Gilead Medical Scientist (MSL), tell us a few details and our team will contact you. You will be contacted for a meeting in approximately 7 business days. If your request is time sensitive, please call Gilead Medical Information (1-866-MEDI-GSI).
Thank you, your request has been submitted.